March 11, 2020
What causes gigantic blobs of hydrogen to glow?
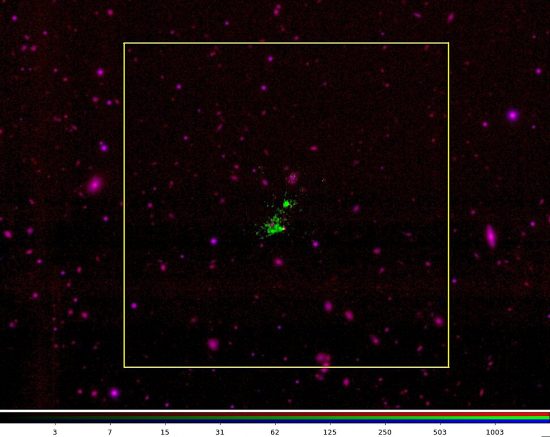
Over ten years ago, Chinese astronomers from Purple Mountain Observatory, Chinese Academy of Sciences, saw what was later called a “Lyman-alpha blob” (LAB). They recognized that there was something unusual about the observation, because of the gas cloud’s mass.
Recently, another group working with the Very Large Telescope (VLT) at the European Southern Observatory (ESO) and the Atacama Large Millimeter/Submillimeter Array (ALMA) announced that (LAB-6), almost 11 billion light-years away, is exceptional because its gas appears to be “falling inward.”
Zheng Zheng, associate professor of physics and astronomy at the University of Utah said:
“This gives us a mystery. We expect there should be infalling gas around star-forming galaxies—they need gas for materials…If the gas had come from this [nearby] galaxy, you should see more metals. But this one, there weren’t a lot of metals.”
What is so unusual about LABS and what does Lyman-alpha mean? Quantum physics postulates that a hydrogen atom’s electron orbit must abide by its principal quantum number. Mathematical calculations use n = 1 for the smallest orbital radius, n = 2 for the next quantized orbital step, n = 3, and so on. As quantum physics postulates, those orbital radii must rise and fall in discrete jumps.
Since electrons are negatively charged, they are attracted to protons by their binding energy. Each “n” orbit’s binding energy is expressed in electron volts. The closer to a hydrogen atom’s nucleus, the greater the binding energy. As an electron jumps down from a higher binding energy orbit to a lower one, it emits light in the ultraviolet range. The photo emissions from the n2 to n1 jump correspond to 121.6 nanometers. This frequency band is known as “Lyman-alpha” radiation, named after Theodore Lyman.
A principal tenet of Electric Universe theory is that electrically active ionized gas, otherwise known as plasma, creates long electromagnetic filaments called Birkeland currents. Gas obeys the laws of kinetic motion, with molecules accelerated by “shock waves” or gravitational attraction. Plasma behaves according to the laws of electricity. Plasma physicists are aware that the anisotropy of an ionized gas will polarize light shining through it. Polarized emissions are sometimes used to map ionized gas and magnetic fields in discrete sources.
Previous Picture of the Day articles repeatedly affirm that when electrons are moving they are called an electric current. An electric current in a magnetic field is defined as, “field-aligned” and can release synchrotron radiation. Lyman-alpha radiation is one way that synchrotron radiation manifests.
The excitation frequency of a specific gas is a more correct model for LABs. Electricity passing through neon gas causes it to glow pale red. Other gases, such as oxygen, produce blue light, while heavier elements have their own colors. In the case of this cosmic blob, electrically excited hydrogen is producing an ultraviolet glare. The conclusions reached by the University’s research are assumptions based on a gravity-only cosmology.
Stephen Smith
The Thunderbolts Picture of the Day is generously supported by the Mainwaring Archive Foundation.